Hippocampal Subfield
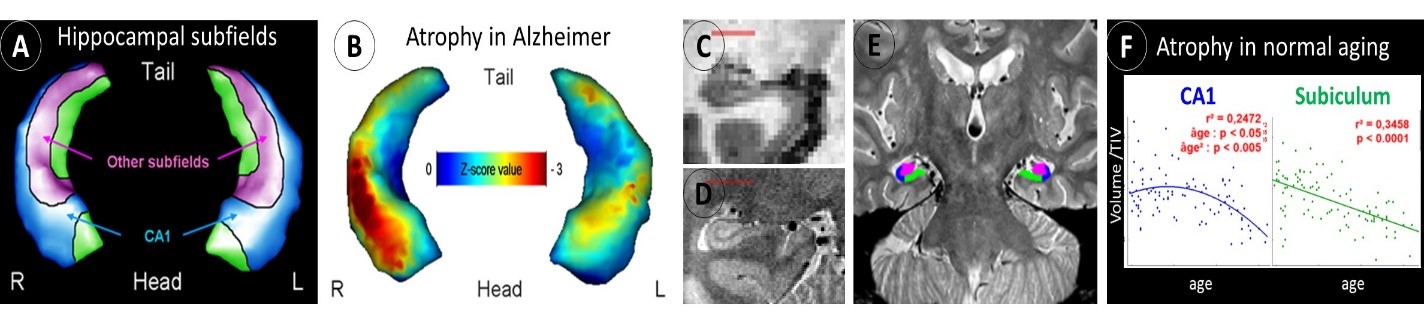
Hippocampal atrophy is well-known as an early biomarker of AD, but it lacks specificity as it is also observed in many different situations other than AD such as normal ageing and other neurodegenerative diseases (eg. fronto-temporal dementia, including semantic dementia (SD)). Neuropathological studies have shown that hippocampal subfields (subiculum, CA1-4 and dentate gyrus) are differentially vulnerable to AD; hippocampal subfield volumetry may thus prove to be more accurate than global hippocampal volumetry to detect AD. This has been confirmed in an early work where we used a voxelwise analyses coupled with 3D hippocampal surface mapping to illustrate the discrepancies between the effects of AD versus normal ageing on hippocampal subfield volumes, with a preferential involvement of the CA1 subfield versus the subiculum, respectively (Chételat et al., 2008) (Figure 1A-B).
The same approach allowed us to illustrate the specificity of the relationships between hippocampal subfield volumes and episodic memory deficits in MCI patients (Fouquet et al., 2012). These findings encouraged us to develop a proton density sequence for very high resolution acquisition of the hippocampus together with guidelines for hippocampal subfield delineation (La Joie et al., 2010) (Figure 1C-D-E). Using this improved technology, we showed the differential involvement of the hippocampal subfields in normal ageing, MCI, AD and SD (La Joie et al., 2010, 2013; de Flores et al., 2015).
Thus, a linear effect of normal ageing was observed on subiculum from 20 to 90 years old, while the effect on CA1 volume was non-linear with a decrease starting from 50 years old only (De Flores et al., 2015) (Figure 1F). CA1 was the most sensitive subfield in early AD with higher accuracy to discriminate between MCI and cognitively normal elderly than the whole hippocampus (La Joie et al., 2013). Semantic dementia was characterized a hemispheric and antero–posterior asymmetry, significantly more marked than in AD, with greater involvement of the left and anterior hippocampal subfields (La Joie et al., 2013).
Altogether, our works highlight the relevance of high-resolution hippocampal acquisition in the early and differential diagnosis of AD. This MRI sequence and related guidelines have been successfully implemented in other research Units who requested our collaboration for this purpose (Wolf et al., 2015). Moreover, we participate in a worldwide collaborative project (http://www.hippocampalsubfields.com/) aiming at standardizing hippocampal subfield delineation (Yushkevich et al., 2015; Wisse et al., 2017) for which we recently obtained a JPND European funding. Recently, we improved our MR sequence to obtain a better resolution without increasing the acquisition time. On-going studies also highlight the relevance of this approach coupled with structural and functional connectivity to further understand the pathophysiology of the disease and more specifically the role of brain connectivity in the topography of the lesions and their propagation.